How to Explain Different Types of Chem
This type of titration majorly depends on the track change in pH or a pH meter. Class C Fires.

Types Of Chemical Bonds Funny Teaching Chemistry Chemical Bond Chemistry Classroom
In a combination reaction two or more elements combine to form a compound or an element and a compound can combine to form a new compound.

. The way that chemists study matter and change and the types of systems that are studied varies dramatically. Explain the different types of chemical bonds and describe how each bonds are formed. Never attempt to extinguish a Class B fire with water.
Draw a sketch of the geometry of NF3 molecule. This is a brief outline of the concept of enthalpy. Organic Analytical Physical Inorganic and Biochemistry.
Corrosive Hazards -Materials that cause visible destruction andor irreversible alterations at point of contact. Indicate in your sketch any lone pairs of electrons on. Each of these types requires different skills and engineering educations.
In broad terms engineering can be divided into four main categories chemical civil electrical and mechanical engineering. Synthesis reaction - A synthesis reaction is one where two substances combine to make a new substance. Magnesium and oxygen combine when heated to form magnesium oxide.
Chemical bonds can be classified into two types as Primary strong bonds- These are the bonds that are strong and difficult to break. There are many types of chemical reactions. Using Lewis dot structures indicate the movement of electrons during the formation of the ionic compound Calcium Oxide CaO from Calcium Ca and Oxygen O.
There are many different types of spectroscopy but the most common types used for chemical analysis include atomic spectroscopy ultraviolet and. 2020 median salary - 108540. If HIn be an indicator a weak acid then its dissociation may be represented as.
The dissociation constant of an acid indicator. A compound is a substance made up of a definite proportion of two or more elements. When a Class B fire sparks extinguish it by smothering the flames.
It can be shown in an equation such that A B -- AB. Ionic bonding It is done between metal and non metalThe metal loses and electron and gives it to the nonmetalFor example Na and Cl- so they make NaCl. A chemical change where one element replaces a second element in a compound.
Class C fires are fires that involve electrical equipment including wiring plug sockets appliances and circuit breakers. Oxidation and reduction reaction. It contains the symbols of the.
However our main aim in this chapter is to earn about the. Typical required education - Bachelors Degree. In this reaction two simple reactants react to.
Oppressing the fire eliminates the oxygen feeding the flames and thereby deadens the fire. The different types of reactions areexamples after the list. Here are a few examples.
Acetonitrile Methanol Diesel Fuel Mineral Spirits. We can calculate the reaction enthalpy by subtracting the sum of enthalpies of all the reactants from that of the products. Types of Chemical Hazards.
Answer 1 of 3. Over the last several years additional concentrations have begun to emerge including Nuclear chemistry Polymer chemistry. Examples of a flammable hazard.
The redox titration is also known as an oxidation-reduction reaction. On the reactant side there is a single element and a compound as there is on the product side. It is identifiable by its symmetry.
Ionised and unionised forms possess different colour. No reaction will occur if. These include like Covalent Ionic and Metallic bonds.
Indicators in chemistry get inside definition function and type of Indicators get the mechanism of Indicators in chemistry and how it is used in titrations. Covalent Bonding It is done between 2 non metals and they both share electrons for example H and OH- and they make H2O. Mathematically Δ t H Sum of enthalpies of the product Sum of the enthalpies of the reactants.
A chemical formula tells us the number of atoms of each element in a compound. Traditionally chemistry has been broken into five main subdisciplines. Dry chemicals like ammonium phosphate or pressurized carbon dioxide are effective means to extinguish a Class B fire.
Secondary weak bonds These bonds are weaker comparatively and are temporary bonds like London dispersion forces Dipole-dipole force and Hydrogen bonding. Decomposition reaction - A decomposition reaction is where a complex substance breaks down to form two separate substances. Flammable Hazards - Materials that burn or ignite.
In this type of titration the chemical reaction takes place with a transfer of electrons in the reacting ions of aqueous solutions.

The 8 Types Of Arrows In Organic Chemistry Explained Master Organic Chemistry Chemistry Chemistry Lessons Organic Chemistry

Chemistry Notes Types Of Chemical Reactions Solution Stoichiometry Chemistry Notes Chemistry Organic Chemistry Notes

Chemical Reactions Types Worksheet Chemical Reactions Types Worksheet Awesome Types Chemical Chemistry Lessons Teaching Chemistry Science Chemistry

6 Main Types Of Chemical Reactions Google Search Chemistry Classroom Chemistry Lessons Chemical Reactions

Picture Teaching Chemistry Chemistry Chemical Reactions
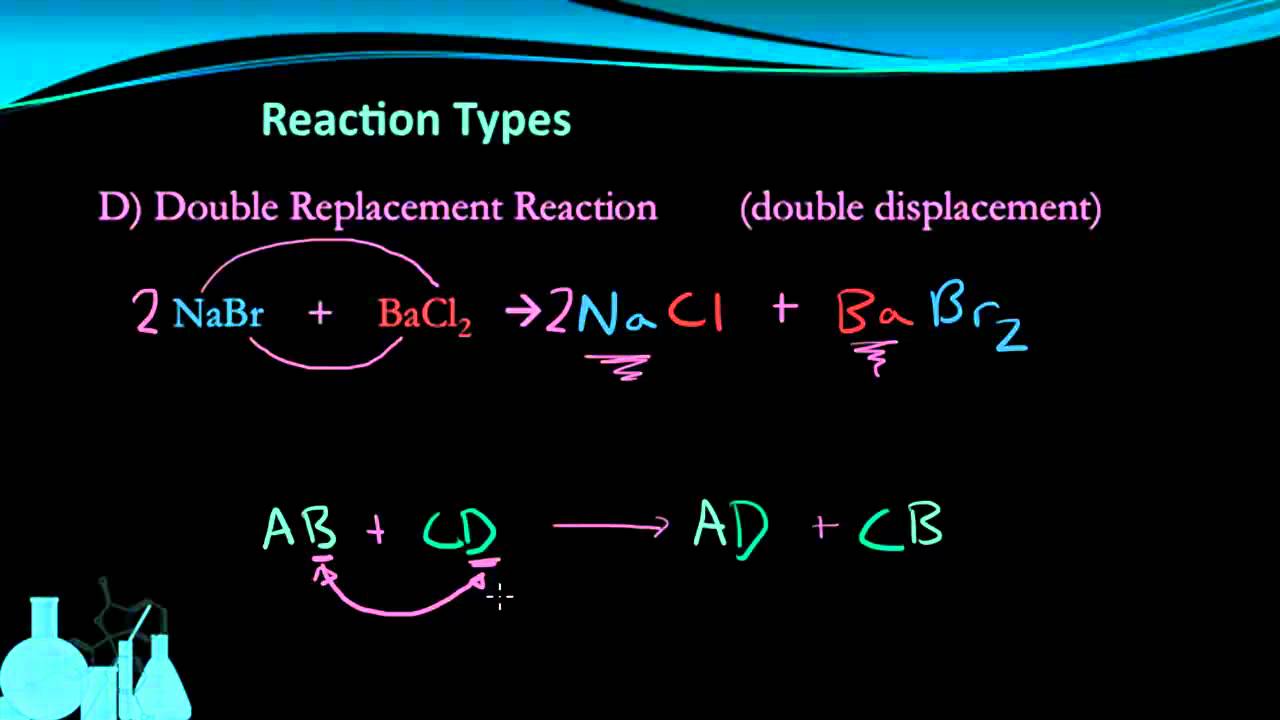
Chemistry Module 5 Types Of Chemical Reactions Http Www Youtube Com Watch V Aawccqb75d0 Single

The 8 Types Of Arrows In Organic Chemistry Explained Master Organic Chemistry Organic Chemistry Organic Chemistry Study Study Chemistry

Introduction To Chemical Reactions Chemistry Lessons Teaching Chemistry Chemistry

Pictorial Representation Of The Five Types Of Chemical Reactions Nicole Galante Chemistry Classroom Teaching Chemistry Chemical Reactions

Igcse Hydrocarbon Classifications Names And General Formula Chemistry Basics Chemistry Gcse Chemistry

Learning How To Predict Products From Different Types Of Chemical Reactions Teaching Chemistry Teaching Science Science Lessons

The 8 Types Of Arrows In Organic Chemistry Explained Master Organic Chemistry Organic Chemistry Chemistry Organic Chem

Basic Chemistry Chemistry Basics Chemistry Classroom Teaching Chemistry

The 8 Types Of Arrows In Organic Chemistry Explained Master Organic Chemistry Organic Chemistry Chemistry Chemistry Help

Learn Chemistry Chemistry Education Chemistry Lessons Chemistry Classroom

Pictorial Representation Of The Five Types Of Chemical Reactions Nicole Galante Chemistry Classroom Teaching Chemistry Chemical Reactions

What Is The Difference Between Organic And Inorganic Chemistry Organic Chemistry Chemistry Education Medical Technology

Types Of Organic Chemistry Formula Poster By Compound Interest In 2022 Organic Chemistry Study Chemistry Classroom Chemistry Basics
